Exploring the world of chemistry, the polarity of the hydrogen sulfide (H2S) molecule holds the key to understanding its behavior and interactions in various chemical processes. In this article, we will delve into the concept of molecular polarity, unraveling the structure of H2S and discovering how to determine the polarity of a molecule. By understanding the polarity of H2S, you’ll gain valuable insights that can help you predict its implications in chemical reactions.
What is Polarity?
Polarity is a fundamental concept in chemistry that describes the unequal distribution of electrons within a molecule. This unequal distribution is caused by differences in the electronegativity of the atoms involved in the chemical bond. Electronegativity is a measure of an atom’s ability to attract shared electrons in a bond.
The polarity of H2S, or the determining molecular polarity, is an important factor in understanding the implications of polarity in chemical reactions. When atoms with different electronegativities form a bond, the shared electrons are not equally distributed, resulting in a partial positive charge on one atom and a partial negative charge on the other.
This unequal charge distribution creates a dipole moment within the molecule, which determines its overall polarity. Understanding the factors that contribute to molecular polarity is crucial for predicting the behavior and interactions of chemicals in various applications.
The Structure of H2S
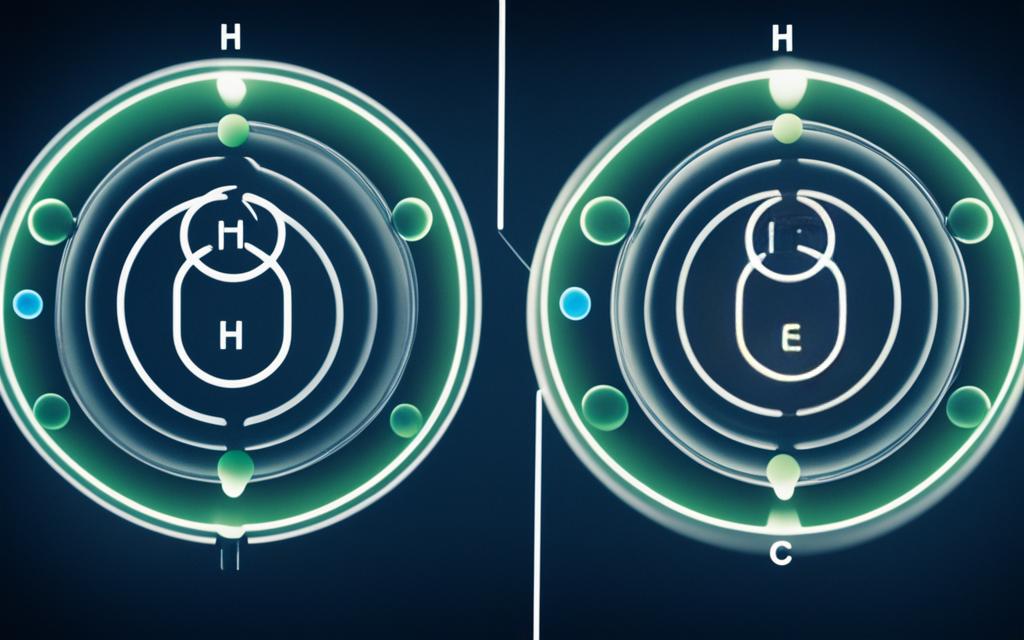
The hydrogen sulfide (H2S) molecule consists of one sulfur (S) atom covalently bonded to two hydrogen (H) atoms. The molecular structure of H2S is bent, with a bond angle of approximately 92 degrees. This bent shape is due to the lone pairs of electrons on the sulfur atom, which occupy more space than the bonding pairs and cause the molecule to adopt a nonlinear geometry.
The polarity of H2S can be determined by considering the electronegativity difference between the sulfur and hydrogen atoms, as well as the molecular geometry. Sulfur has a higher electronegativity than hydrogen, which means that the shared electrons in the S-H bonds are pulled more towards the sulfur atom, resulting in a partial negative charge on the sulfur and a partial positive charge on the hydrogen atoms.
Understanding the molecular structure and polarity of H2S is crucial for predicting its behavior and interactions in various chemical processes. The implications of polarity in chemical reactions can have significant consequences, affecting the solubility, reactivity, and other properties of the molecule.
Is H2S Polar or Nonpolar?
To determine the polarity of the H2S molecule, we need to consider the electronegativity difference between the sulfur and hydrogen atoms, as well as the molecular geometry. Sulfur has a higher electronegativity than hydrogen, which means that the shared electrons in the S-H bonds are pulled more towards the sulfur atom, resulting in a partial negative charge on the sulfur and a partial positive charge on the hydrogen atoms.
The bent molecular geometry of H2S, with a bond angle of approximately 92 degrees, also plays a crucial role in determining its polarity. This bent shape is due to the lone pairs of electrons on the sulfur atom, which occupy more space than the bonding pairs and cause the molecule to adopt a nonlinear geometry.
Given the unequal distribution of electrons within the H2S molecule and its nonlinear geometry, the partial charges on the sulfur and hydrogen atoms cancel each other out, resulting in an overall nonpolar molecule. This means that the H2S molecule is considered nonpolar, despite the polarity of the individual bonds.
| Characteristic | Explanation |
|---|---|
| Electronegativity Difference | Sulfur has a higher electronegativity than hydrogen, leading to an unequal distribution of electrons in the S-H bonds. |
| Molecular Geometry | The bent shape of the H2S molecule, with a bond angle of approximately 92 degrees, cancels out the partial charges, resulting in a nonpolar molecule. |
| Overall Polarity | Despite the polarity of the individual bonds, the H2S molecule is considered nonpolar due to its molecular geometry. |
Polarity and Chemical Properties
The polarity of H2S has significant implications for its chemical properties and behavior. Polar molecules, such as water (H2O), tend to be more soluble in polar solvents and can participate in hydrogen bonding and other intermolecular interactions. In contrast, nonpolar molecules like H2S are typically more soluble in nonpolar solvents and do not engage in hydrogen bonding to the same extent as polar molecules.
The implications of polarity in chemical reactions are far-reaching. Polar molecules often exhibit higher reactivity and can participate in a variety of chemical processes, such as acid-base reactions, nucleophilic additions, and solvation. Nonpolar molecules, like H2S, may have different reactivities and often exhibit weaker intermolecular forces, which can affect their physical properties, such as boiling point and solubility.
Understanding the determination of molecular polarity is crucial for predicting the behavior and interactions of chemical species in various applications, from organic synthesis to biochemistry. By considering the electronegativity differences and molecular geometry, chemists can accurately determine the polarity of a molecule and anticipate its potential chemical properties and interactions.
Determining Molecular Polarity
Determining the polarity of a molecule, such as hydrogen sulfide (H2S), involves considering the electronegativity difference between the atoms involved in the chemical bonds and the overall molecular geometry. Molecules with a significant electronegativity difference and a nonlinear geometry, like H2S, are often nonpolar. On the other hand, molecules with a large electronegativity difference and a linear or trigonal planar geometry tend to be polar.
The polarity of a molecule is directly related to the distribution of electrons within the chemical bonds. When there is a significant difference in electronegativity between the atoms, the shared electrons are pulled more towards the atom with the higher electronegativity, creating a partial positive charge on one side and a partial negative charge on the other. This unequal distribution of charges is what gives the molecule its polarity.
In the case of H2S, the sulfur atom has a higher electronegativity than the hydrogen atoms, resulting in a partial negative charge on the sulfur and a partial positive charge on the hydrogen atoms. However, the bent molecular geometry of H2S, with a bond angle of approximately 92 degrees, causes the partial charges to cancel out, making the overall molecule nonpolar.
Understanding the factors that determine molecular polarity, such as electronegativity and molecular geometry, is crucial for predicting the behavior and interactions of molecules in various chemical processes. This knowledge helps chemists and scientists anticipate the solubility, reactivity, and other properties of compounds, ultimately aiding in the development of new materials and the optimization of existing chemical systems.
Conclusion
Throughout this exploration of the polarity of H2S, we’ve gained a deeper understanding of the factors that determine molecular polarity and their implications in chemical reactions. The bent molecular geometry of hydrogen sulfide, combined with the difference in electronegativity between sulfur and hydrogen, results in a nonpolar molecule.
This knowledge of determining molecular polarity is crucial for predicting the behavior and interactions of H2S in various chemical processes. As a nonpolar molecule, H2S exhibits distinct implications for its chemical properties, such as solubility and intermolecular interactions, compared to polar counterparts.
By delving into the intricacies of molecular polarity, we’ve gained a more comprehensive understanding of the fundamental principles governing the structure and behavior of chemical compounds like H2S. This knowledge can be applied to a wide range of scientific disciplines, from chemistry and physics to materials science and environmental studies.